As enema machines become increasingly advanced and integrated into clinical and home care environments, the design of their power supply systems has become more crucial than ever. A poorly designed power adapter can introduce electromagnetic interference (EMI), pose safety risks, or reduce system longevity. In this article, we’ll dive deep into the key considerations for power supply design in enema machines—from electromagnetic compatibility (EMC) to leakage protection, covering technical standards, real-world application challenges, and design optimization strategies.
1. Introduction: The Role of Power Adapters in Medical Equipment
Enema machines, while relatively simple in function, must adhere to stringent safety, reliability, and hygiene requirements due to their close contact with patients. The external power supply unit (PSU), commonly a switching power adapter, plays a pivotal role in:
2. Understanding Electromagnetic Compatibility (EMC) in Enema Machines
2.1 What is EMC?
Electromagnetic compatibility refers to a device’s ability to function correctly in its electromagnetic environment without introducing interference to other systems.
2.2 Why is EMC critical in medical environments?
- Interference with other medical devices: Hospitals are filled with sensitive diagnostic and therapeutic equipment.
- Compliance requirements: Standards such as IEC 60601-1-2 mandate EMC thresholds for medical devices.
- Patient safety: High-frequency switching noise can disrupt patient monitoring systems.
2.3 Design strategies for EMC compliance
- Use of ferrite beads, common mode chokes, and EMI filters
- Shielded enclosures for adapters and cables
- Optimized PCB layout to reduce loop areas and minimize radiated emissions
- Compliance testing in anechoic chambers
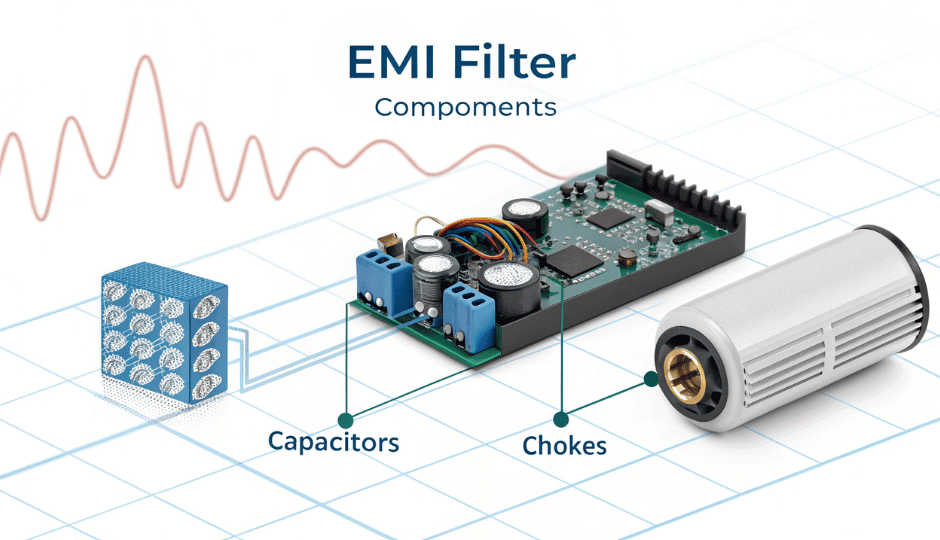
3. Leakage Current: Managing Risk in a Moisture-Rich Application
3.1 What is leakage current?
Leakage current is the unintended flow of electric current through a device’s insulation or protective earth ground. In medical equipment, it poses a significant safety concern.
3.2 Why leakage protection matters for enema machines?
- Enema machines often operate near water sources.
- Patients may be in contact with conductive surfaces.
- Even low leakage current can cause discomfort or harm.
3.3 Medical standards and limits
According to IEC 60601-1, leakage current for Type BF applied parts must not exceed:
- Normal condition: ≤ 100 µA
- Single fault condition: ≤ 500 µA
3.4 Mitigation strategies
- Double or reinforced insulation
- High-quality Y-capacitors with medical-grade ratings
- Transformer designs with low parasitic capacitance
- Ground isolation with proper creepage/clearance distances
4. Isolation and Insulation Requirements
4.1 The importance of galvanic isolation
- Prevents DC path between AC mains and patient-contact circuits
- Ensures safety during power surges or ground loops
4.2 Medical-grade isolation standards
- Minimum 2xMOPP (Means of Patient Protection) between input and output
- Isolation voltage: typically 4,000–5,000 V AC
- Dielectric strength testing during QC procedures
5. Safety Certifications and Regulatory Compliance
5.1 Global medical power adapter certifications
- UL 60601-1 (USA)
- EN 60601-1 (Europe)
- CSA C22.2 No. 60601-1 (Canada)
- CB Scheme for international approval
5.2 Key documents and reports
- EMC test reports
- Safety test reports (hi-pot, insulation resistance)
- Risk Management File (per ISO 14971)
5.3 Labeling and traceability
- Clear markings for voltage/current ratings
- Serial numbers for product traceability
- Warning labels on the adapter casing
6. Environmental and Durability Considerations
6.1 Thermal performance
Enema machines often operate for extended periods. The power supply must:
- Avoid overheating under full load
- Use efficient topologies (e.g., flyback, LLC resonant)
- Incorporate temperature protection features
6.2 IP rating and ingress protection
Depending on usage:
- Enema machine power adapters may require an IPX1 to IPX4 rating
- Splash-proof or waterproof enclosures using sealed connectors
6.3 Long-term reliability
- Industrial-grade capacitors (≥ 105°C, long lifespan)
- Surge protection (TVS diodes, MOVs)
- Conformal coating to prevent moisture damage
7. Power Requirements and Adapter Selection
7.1 Power rating
Typical enema machines require power between 12W–60W, depending on their:
- Motor/pump size
- Heating elements
- User interface/display
7.2 Output voltage standards
Common voltages:
- 12V DC
- 24V DC
7.3 Connector type and polarity
- Secure locking connectors to prevent accidental unplugging
- Standard barrel plug with positive center
8. Benefits of Using External Power Adapters
8.1 Design modularity
- Easy replacement or upgrades
- Simplified internal electronics layout
8.2 Reduced thermal stress
- Heat is dissipated externally
- Enhances device lifespan
8.3 Easier global certification
- External adapters can be certified separately
- Reduces cost/time for product redesign
9. Emerging Trends in Medical Power Supply Design
9.1 GaN-based adapters
- Higher efficiency
- Smaller size
- Better thermal performance
9.2 Smart power supplies
- Built-in diagnostics
- Communication interfaces (e.g., UART, I²C)
- Remote monitoring for predictive maintenance
9.3 Environmentally conscious design
- Compliance with RoHS, REACH
- Energy efficiency standards (DoE Level VI, EU CoC Tier 2)
10. Conclusion: Optimizing for Safety and Performance
Designing power adapters for enema machines is far more than selecting an off-the-shelf PSU. It requires an in-depth understanding of medical safety, EMI control, leakage management, and regulatory compliance. By prioritizing EMC, insulation, and leakage protection from the very beginning, manufacturers can ensure a safer, longer-lasting, and globally compliant product.
Glossary
- EMC: Electromagnetic Compatibility
- EMI: Electromagnetic Interference
- PSU: Power Supply Unit
- BF Type: Body Floating Applied Part
- MOPP: Means of Patient Protection
- UL/EN/IEC/CSA: Safety standards bodies and certifications
References
- IEC 60601-1: Medical electrical equipment – General requirements for basic safety and essential performance
- IEC 60601-1-2: EMC requirements
- ISO 14971: Application of risk management to medical devices