Introduction
The safety and reliability of medical electrical equipment are directly linked to patient health and even survival. As a globally recognized “golden standard,” the IEC 60601 series has guided the medical device industry’s safety framework and market access requirements since its first publication in 1977. Among all medical devices, the power adapter is a critical yet often overlooked component. It not only supplies stable power but also ensures overall safety and performance. Without compliance with IEC 60601, even the most advanced medical device cannot enter the market. This article reviews the evolution of IEC 60601, with a focus on the transformational shift from the second to the third edition, and explains the profound implications for medical power adapters with practical case examples.
Historical Development of IEC 60601
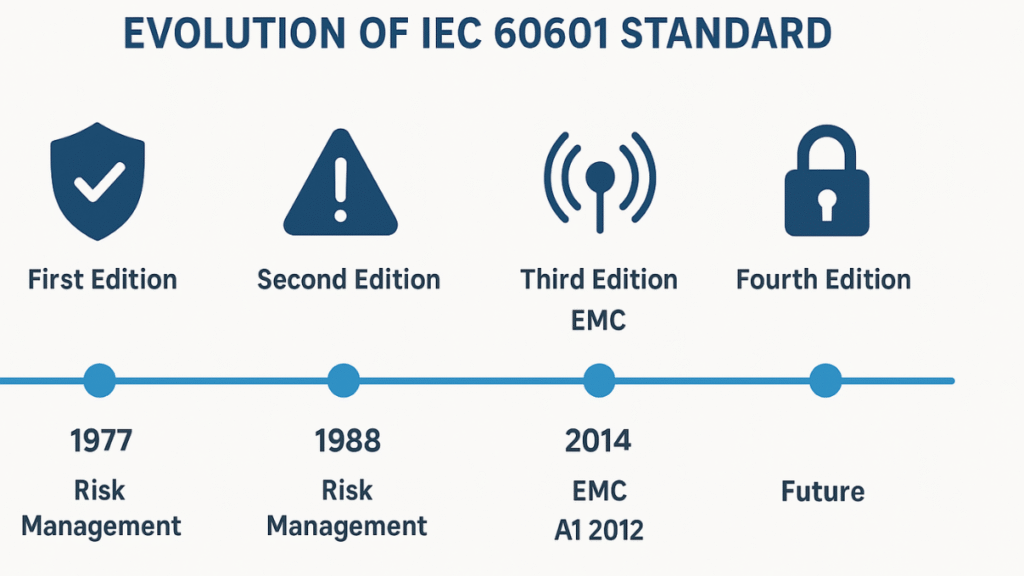
First Edition (1977)
The IEC 60601-1 first edition introduced a unified international safety standard for medical electrical equipment, with its core emphasis on electrical safety. It defined concepts such as “patient leakage current” and “touch current.” For power adapters, this meant strict control of electrical parameters during design and production to prevent harm to patients via the power path.
Second Edition (1988)
The second edition, published in 1988, significantly expanded the scope. Beyond electrical safety, it introduced mechanical safety requirements such as stability, drop resistance, and impact endurance, as well as environmental factors like heat and humidity resistance.
For power adapters, this edition required:
- Stricter leakage current limits and insulation design to ensure patient protection.
- Improved environmental adaptability, ensuring reliable operation in complex hospital environments.
Third Edition (2005, Amendment A1 in 2012)
As medical technology advanced toward integration and intelligence, safety alone was no longer sufficient. The 2005 third edition became a milestone by introducing the concept of Essential Performance, emphasizing that equipment must maintain its core clinical function under all conditions.
Most importantly, it formally integrated risk management based on ISO 14971, requiring manufacturers to systematically identify and mitigate potential risks. For medical power adapters, this introduced major challenges:
- Stable power delivery: adapters must maintain performance under voltage fluctuations, transient overload, or external interference.
- Enhanced insulation and leakage current control, leading to the use of reinforced isolation and double insulation structures.
- Software and intelligent control: adapters incorporating microcontrollers or communication modules must follow IEC 62304 requirements for software lifecycle safety.
- Usability considerations: aligned with IEC 62366, adapter indicators, connectors, and labels must be designed to prevent misuse.
The 2012 Amendment A1 expanded the scope to home-use medical equipment, requiring adapters to handle unstable residential grids and higher levels of electromagnetic interference.
Fourth Edition (2014, IEC 60601-1-2 EMC)
The 2014 IEC 60601-1-2 fourth edition focused on electromagnetic compatibility (EMC). In an era of pervasive wireless communication, it requires equipment to withstand stronger electromagnetic disturbances. For power adapters, this means:
- Passing stricter immunity and emission tests.
- Avoiding electromagnetic emissions that could disrupt other devices.
- Maintaining stable voltage output even in environments with WiFi and Bluetooth interference.
Future Outlook
The upcoming fourth edition of IEC 60601-1 is expected to address cybersecurity, AI algorithm safety, energy efficiency, and sustainability. For adapters, this implies additional requirements for resilience against cyber-related risks and compliance with higher energy efficiency standards for sustainable healthcare.
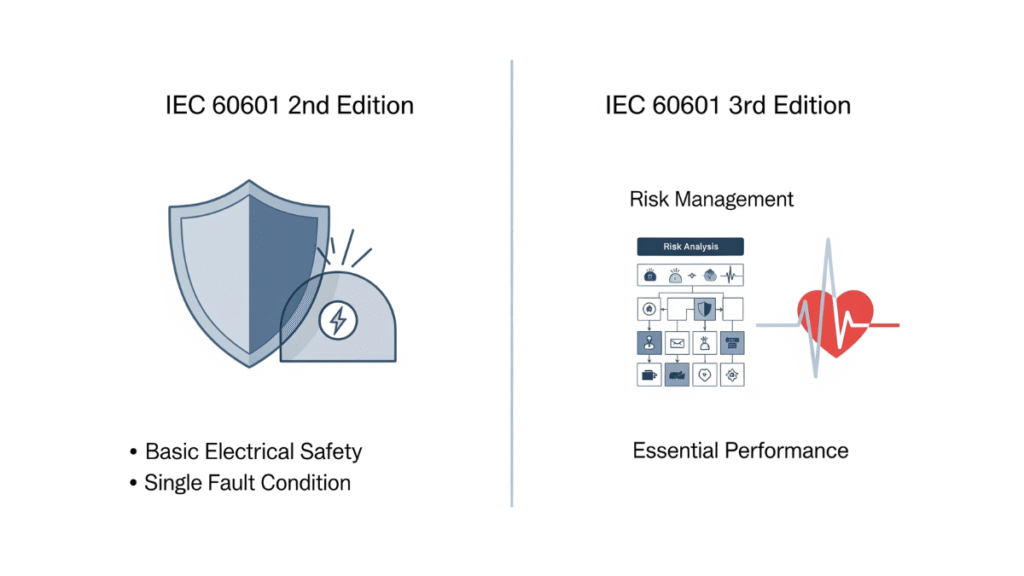
Key Changes from the Second to the Third Edition: Implications for Power Adapters
The transition from the second to the third edition represents the most significant paradigm shift in IEC 60601 history:
- From Safety Alone → Safety + Performance Assurance
While the second edition focused on “avoiding harm,” the third edition requires that equipment must also maintain functionality during critical moments. Power adapters must therefore not only protect against electric shock but also ensure uninterrupted supply under real-world challenges. - Compliance Methods
Under the second edition, compliance relied heavily on physical testing. The third edition requires additional documentation, including risk management files, design verification, and reliability evaluations. - Impact on Design and Manufacturing
Power adapter manufacturers must now integrate ISO 14971 risk management during R&D, covering circuit design, component selection, redundancy planning, and more—raising both design complexity and quality assurance standards. - Wider Application Scenarios
With A1, home-use medical devices were added, meaning adapters must work reliably not only in hospital environments but also in households with unstable grids and stronger wireless interference.
Real-World Case Examples
- Patient Monitors
Continuous operation is critical for patient monitors. Power interruptions can disrupt vital sign tracking. IEC 60601-1 third edition-compliant adapters, with tightly controlled leakage current and stable voltage, ensure uninterrupted monitoring, even under fluctuating power supply conditions. - Ultrasound Systems
Diagnostic accuracy in ultrasound depends on high-quality imaging. A poorly designed power adapter may cause electromagnetic interference, distorting images. By meeting IEC 60601-1-2 fourth edition EMC requirements, compliant adapters minimize noise and guarantee precise imaging results. - Ventilators
For ICU ventilators, power loss can endanger lives. Third edition-compliant adapters feature redundancy, overload protection, and ultra-low leakage current, ensuring stable and safe operation in critical care environments. - Home-Use Devices (e.g., glucometers, portable nebulizers)
With A1 extending to home use, adapters must withstand unstable residential power grids and heavy wireless interference. High-quality medical adapters therefore integrate advanced EMI filters and reinforced insulation to ensure safe, accurate operation at home.

Conclusion
The evolution of IEC 60601 reflects the medical industry’s ongoing pursuit of safety, reliability, and performance. For power adapters, compliance is not just a regulatory requirement but a mark of product quality and market readiness. The transition from the second to the third edition marked a shift from basic safety compliance to comprehensive risk management and performance assurance.
As IEC 60601 continues to evolve, medical power adapters will need to advance in electrical safety, risk management, electromagnetic compatibility, cybersecurity, and energy efficiency. Whether in hospital monitoring systems, diagnostic imaging devices, life-support ventilators, or home medical applications, compliant adapters will remain the foundation of safe and reliable healthcare delivery worldwide.




